[ad_1]
Antibiotic resistance may lead to more coronavirus deaths
Doctors knew antibiotics weren’t effective against the coronavirus, but they feared patients could develop life-threatening bacterial co-infections and used them anyway, raising concerns about antibiotic resistance. Dr. Jeffrey Strich, a researcher and physician at The National Institutes of Health Clinical Center sits down with Fox News Digital to explain how the COVID-19 pandemic has contributed to the mounting resistance to antibiotics and how this plight will raise the death toll in the United States.
When the coronavirus hit the United States in March, doctors were left scrambling on how to treat infected patients. With no drug proven for treating COVID-19 patients, many turned to antibiotics, medications typically used to fight bacterial infections.
Dr. Jeffrey Strich, a researcher and physician at The National Institutes of Health Clinical Center, says most patients were getting antibiotics at some point during their hospitalization.
“It’s very difficult, particularly when you’re learning about a disease for the first time, to know whether this is the right thing or wrong thing to do. But when you have patients who are very sick in front of you. You want to do everything you can do to help them,” Strich told Fox News.
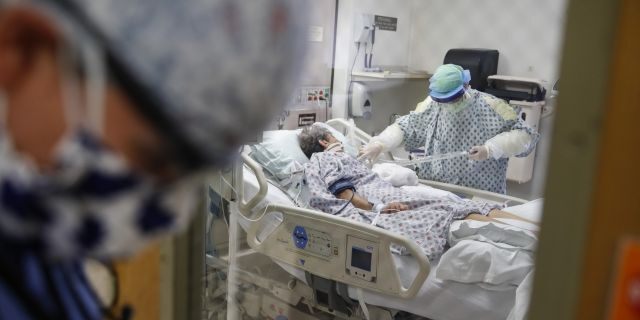
Doctors knew antibiotics weren’t effective against viruses like COVID-19, but they feared patients could develop life-threatening bacterial co-infections and used them anyway, raising concerns about antibiotic resistance.
Secondary infections could include community-acquired pneumonia and urinary tract infections, among others, Strich said. Hospital-associated infections can be particularly resistant to different antibiotics as well, he added. Such infections include ventilator-associated pneumonia, a central line-associated bloodstream infection, or urinary tract infections associated with catheters.
FISH MUCUS SHOWS PROMISE AS ANTIBIOTIC, EVEN AGAINST SUPERBUGS
A recent study found 50 percent of patients with COVID-19 who have died had secondary bacterial infections. The same study also reported on the high use of antibiotics. Study authors discovered nearly 75 percent of COVID-19 patients who are admitted to intensive care units received antimicrobial medicine.
According to the Centers for Disease Control and Prevention (CDC), antibiotic resistance happens when germs like bacteria and fungi develop the ability to defeat the drugs designed to kill them. The agency says infections caused by antibiotic-resistant germs are difficult — and sometimes impossible — to treat.
Some experts believe the growing global threat of antibiotics resistance is considered to be a greater health threat than the COVID-19 pandemic. A 2019 United Nations report estimated that globally, 10 million people may lose their lives by 2050 due to infections resistant to antibiotics.
Strich recently published a study in Lancet Infectious Diseases that looked at the growing need for new antibiotics to treat drug-resistant infections.
“Innovation is not currently sustainable, and the current reimbursement models rely on reimbursement of sales that are volume-based sales of these different antibiotics,” Strich said. “The concern is that drug companies are leaving this base because of the low overall number of infections that would need new drugs. And because of that, the antibiotic pipeline is kind of dwindling away.”
Declining financial returns have caused some antibiotic startups like Achaogen and Aradigm to declare bankruptcy and have driven several large pharmaceutical companies from the market.
“The main conclusion of our study is that the current volume-based sales revenue strategies that are used to sustain the antibiotic market are not sustainable and that we’re going to have to come up with innovative ways to reinvigorate the antibiotic pipeline,” Strich explained.
CORONAVIRUS ANTIBODIES START TO DECREASE ‘WITHIN 2 TO 3 MONTHS’ FOLLOWING COVID-19 INFECTION: STUDY
World Health Organization (WHO) director-general Tedros Adhanom Ghebreyesus told reporters at a virtual press conference from the WHO’s Geneva headquarters on June 1 that the coronavirus pandemic “has led to an increased use of antibiotics, which ultimately will lead to higher bacterial resistance rates that will impact the burden of disease and deaths during the pandemic and beyond.”
Ghebreyesus also stated that the world is losing its ability to use “critically important antimicrobial medicines.”
From a child’s ear infection and strep throat to pneumonia and tonsillitis, antibiotics help treat millions of common infections every day, which makes it clear how important it is to reduce the impact and limit the spread of resistance.
“What we really want to do is make sure that we stay ahead of the game with development of novel antibiotics, with new mechanisms of action, and be one step ahead of the bacteria because the bacteria are always mutating, acquiring other resistance mechanisms that will lead to antibiotic resistance. And there is no way of stopping that,” Strich said. “What we can do is be prepared, have an innovative pipeline, have novel mechanism antibiotics and be one step ahead of the bacteria.”
[ad_2]
Source link

