[ad_1]
As coronavirus testing in the U.S. continues to lag behind that in other highly affected countries such as South Korea, several domestic startups are reportedly launching the first at-home tests.
The products have been greenlit by the U.S. Food and Drug Administration under new expedited guidelines to help combat the virus, according to Stat, a health care industry news outlet.
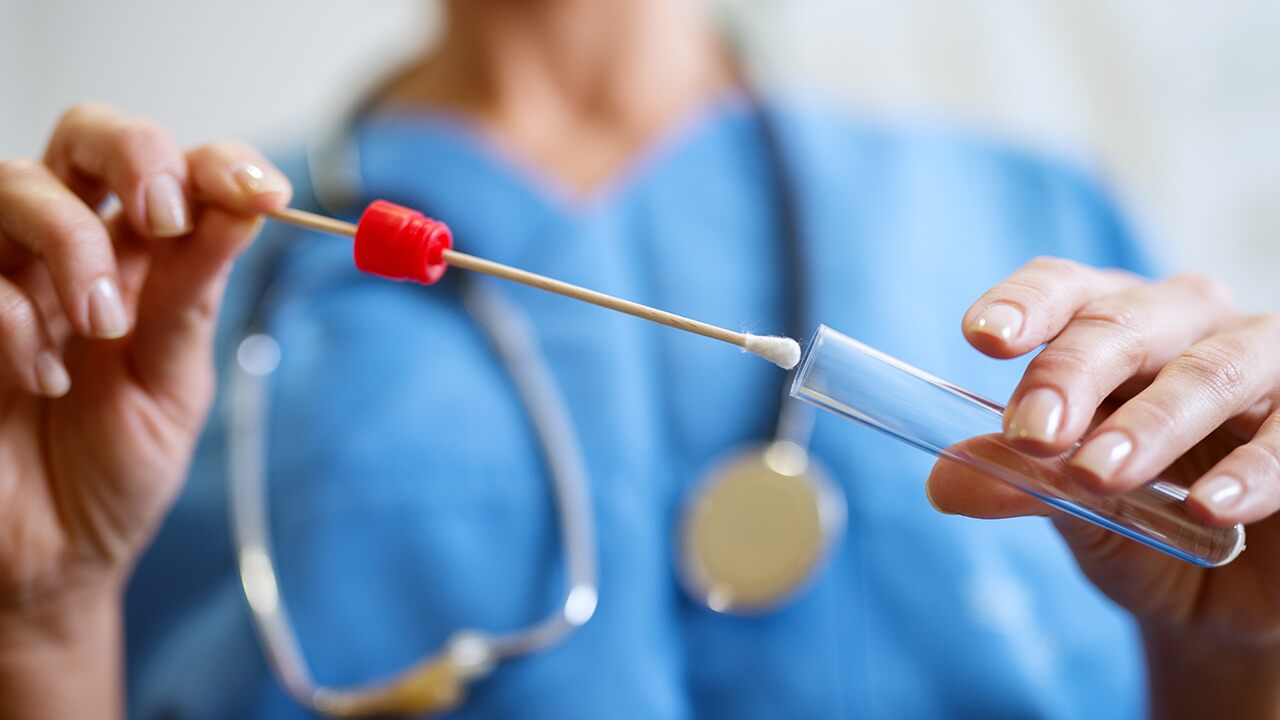
One such test provider is Nurx, a San Francisco-based company best known for home testing products for sexually transmitted infections and birth control, now offers testing kits for COVID-19 through the mail for $181 after prospective customers fill out an online form with their symptoms.
WHO IS STEPHEN HAHN, FDA COMMISSIONER?
“We don’t look for the severity of symptoms, only the presence of them. They can range from mild to severe,” Nurx spokeswoman Allison Hoffman told SFGate.com. “But, people with truly severe symptoms should also seek immediate care. … In terms of temperature in particular, we determine a fever is 100.4 [degrees] plus.”
After filling out the online survey, Nurx will determine if testing is necessary based on the person’s symptoms and will mail the test via expedited shipping.
Nurx can give customers lab results within 48 hours of receiving the test, SFGate reported.
“We are pivoting from our prior service lines to make this available,” Chris Hall, senior clinical adviser for Nurx, told Stat. “It’s a heavy lift, but we think it’s important.”
By Friday evening, Nurx’s website said it had hit capacity and would reopen Saturday morning.
Accuracy concerns
As the tests have rolled out quickly amid the crisis, concerns about their accuracy have also emerged, Stat reported.
Tests not only have to be done at the right time but samples also have to be taken from the right place in the nose and throat.
STARS GET CORONAVIRUS TESTS, RAISING CONCERNS OF INEQUALITY
The FDA also typically “takes a good hard look at the information sent to the consumer,” said Hank Greely, director of the Stanford Center for Law and the Biosciences and the Stanford Program in Neuroscience and Society. “I suspect it hasn’t done that here for lack of time.”
Eric Topol, director of the Scripps Translational Science Institute, said the chance for false negatives is high.
“It’s not a very comfortable thing, if you do it right. It can feel like you’re trying to gag yourself,” he said.
Nurx told Stat that the company’s test uses an oral swab that’s more user-friendly.
Topol added, in his view, 48 hours is too long for people to wait for test results.
“In that time, the person doesn’t know whether they should be hunkering down or doing something else,” he said, according to Stat.
Oregon Dem Wyden warming to GOP plan for coronavirus relief checks, Kudlow says
The startups, however, say their tests have gone through the necessary steps for consumer readiness, Stat reported.
“You might not be sick enough to go to the ER, you might not have severe shortness of breath, but you have cold and flu symptoms and you live with your mom who’s 75 and has heart problems. And you know that if you have it, there’s a chance you could kill her,” Caesar Djavaherian, co-founder and medical director of primary care startup Carbon Health, told Stat. “You might be in your 20s. You won’t die from this. It’s the impact on your family — that’s what we’re trying to lessen.”
Everlywell, an Austin, Texas-based medical diagnostics company, is also rolling out a home test that should have a supply of 30,000 units available by Monday.
“We plan to eventually have testing and diagnosis capacity for a quarter of a million people weekly. However, this process will take several weeks and could take a few months,” the company told USA Today.
CLICK HERE FOR COMPLETE CORONAVIRUS COVERAGE
The startups could also be limited by the supplies available to make the tests.
“The concerns about the supply chain are real concerns,” said Robert Mordkin, U.S. medical director for LetsGetChecked, which is also rolling out a test, according to Stat. “It’s really about what the supply chain will be able to manage, and we don’t know what that number is, quite frankly.”
By Friday afternoon, SFGate reported, at least 138,500 people had been tested for the virus in the U.S. and more than 19,000 had been found to have it.
[ad_2]
Source link