[ad_1]
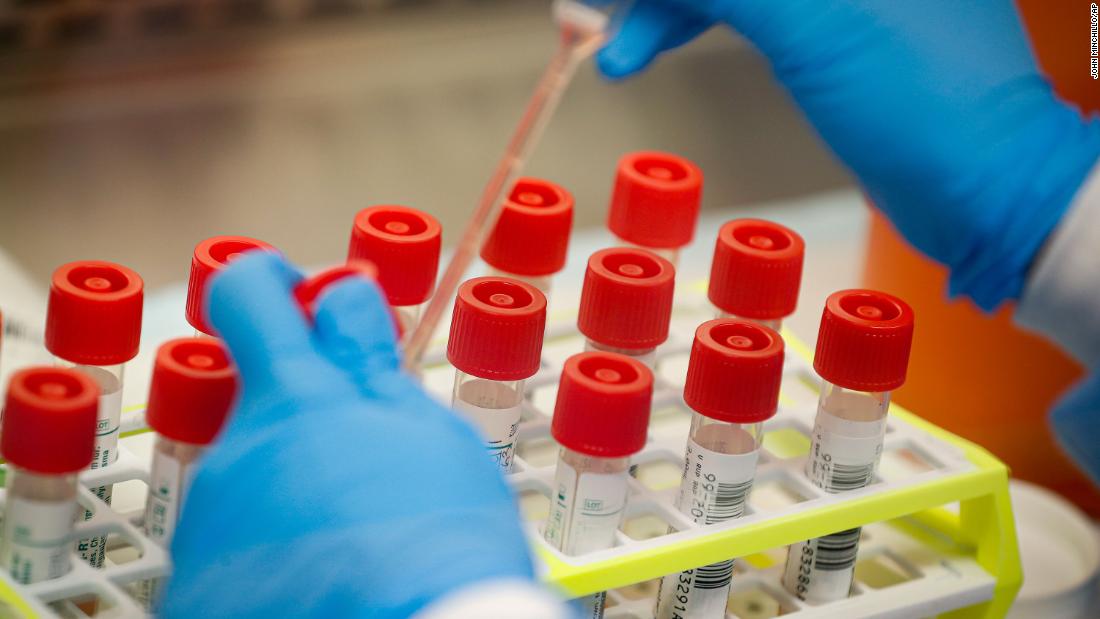
Federal health officials on Friday green-lit a point-of-care coronavirus test that can provide results in less than 15 minutes, using the same technology that powers some rapid flu tests.
Teased by Vice President Mike Pence in a Thursday press briefing, the new diagnostic could accelerate testing in the United States, allowing for rapid results in doctors’ offices. But shortages of critical equipment used to collect patient specimens, such as masks and swabs, could blunt its impact.
The US Food and Drug Administration authorized the test for emergency use, signaling that federal regulators were satisfied with the test’s validation data and believe its benefits outweigh any risks, such as false positives or negatives.
The test’s maker, Abbott, said it expects to deliver 50,000 tests per day beginning next week. The technology behind the test looks for genes that are present in the virus, similar to PCR tests already on the market.
The platform used to run the test weighs less than 7 pounds, according to Abbott, and could be deployed “where testing is needed most,” such as at coronavirus hotspots.
More on this: Last week, the FDA authorized another rapid test – one from molecular diagnostics company Cepheid, which provides results in about 45 minutes. Most laboratory tests for the coronavirus take anywhere from a few hours to days to receive results.
All FDA-authorized tests, however, require samples from patients – and health care facilities say they’re facing shortages of critical supplies needed to collect specimens.
The US Centers for Disease Control and Prevention on Tuesday issued guidance allowing some patients to collect their own nasal swabs in health care facilities, which could reduce the amount of protective equipment needed for health care workers.
But some jurisdictions, such as New York City, have said that patients with coronavirus-like illness should stay home – saying that is “safer for the patients and health care workers” and doesn’t change the treatment patients receive.
[ad_2]
Source link